Promotional Features
How functional acids with added value are improving effervescent beverage tabs
Various dry products utilize active ingredients in highly concentrated forms, resulting in reduced bulk, transportation, and packaging requirements.
This not only minimizes the carbon footprint of the product, but also improves its overall sustainability. Citric acid, a bio-based and biodegradable organic acid, is extensively used in effervescent powders and tablets, including vitamin and mineral supplements, sports drinks, or electrolyte drinks.
In addition to these established product types, there is a growing trend towards innovative beverage tablets that offer sustainable formats and more environmentally friendly packaging options. However, the formulation of effervescent systems presents significant challenges for manufacturers, particularly regarding the production and storage of the final products.
Effervescent systems typically consist of citric acid (C6H8O7) and sodium bicarbonate (NaHCO3). When these components come into contact with water, an effervescent reaction occurs, releasing carbon dioxide (CO2) and creating fizz-like bubbles that rise to the surface.1,2
One major challenge with effervescent systems is their hygroscopic nature, making them sensitive to moisture. Absorption of water during processing and storage can trigger an undesired premature effervescent reaction, resulting in tablet mottling, changes in tablet hardness, and dissolution issues.3
Fortunately, these formulation challenges can be overcome by modifying the particle surface of citric acid through coating or agglomeration processes. This modification enhances the acid’s performance and stability, ensuring optimal results in effervescent beverage tablets.
Functional acids with added value in effervescent beverage tabs
Each product in the functional acids range is designed to tackle specific challenges to enhance processability during production and improve the properties of the final product:
Citric Acid DC A directly compressible citric acid coated with a thin layer of maltodextrin on the surface of its particles which eliminates the requirement for agglomeration before the tableting process, thus saving time and energy.
CITROCOAT® N This is the trade name for citric acid with a monosodium citrate coating by Jungbunzlauer. It is less hygroscopic and less reactive with other ingredients compared to regular citric acid.
CITROCOAT® EP An agglomerated effervescent compound that combines citric acid and sodium bicarbonate in the correct composition to create a highly reactive but storage-stable effervescent powder with a target pH of 5.5.
Performance of effervescent systems
In experiments comparing regular citric acid and Jungbunzlauer’s functional acids in effervescent formulations, the performance of effervescent systems has been assessed.
Powder flow and segregation A homogeneous mixture is essential to ensure the desired reproducible effervescent reaction. Non-uniform mixtures can arise through segregation, influencing the product quality (e.g. composition in bulk, a sachet or a tablet). Segregation was investigated using a Sifting Segregation Tester. CITROCOAT® EP, as a ready-to-use compound, maintained a constant composition during pre-processing or tableting, preventing segregation. On the other hand, regular citric acid and sodium bicarbonate required additional processing to adjust bulk particle sizes and prevent segregation.
Tableting characteristics Good compressibility of the powder mixture is crucial for tablet applications. Agglomeration to improve compressibility is difficult because water-based processes would cause premature reaction of the effervescent components.
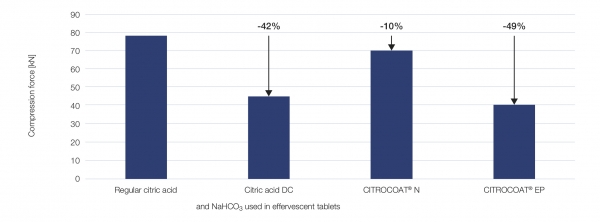
Mixtures of acids and sodium bicarbonate were compressed on a hydraulic single-punch press (FlexiTab® by Röltgen, Solingen, Germany). The results show that functional acids enable tablets of comparable hardness to be produced at lower compression forces. In particular, CITROCOAT® EP enabled tablets of comparable hardness to be produced with 43% less compression force (Figure 1).
Figure 1: Compression forces required for different effervescent mixtures (with additional lubrication using 2 wt% PEG 4000) to achieve comparable tablet hardness of 75±10 N. Percentage reductions in compression force are given relative to the regular citric acid mixture.
Dissolution time of effervescent tablets The dissolution time of an effervescent tablet is an important characteristic. All tested tablets dissolved easily, with the use of functional acids resulting in faster dissolution. The CITROCOAT® EP tablets dissolved in half the time compared to regular citric acid. Furthermore, CITROCOAT® EP tablets had consistent dissolution times due to their homogeneous composition.
Storage stability of effervescent systems While high reactivity is desirable when an effervescent system is in use, it is not desired during storage. The goal is to prevent premature reactions, as this would lead to a loss of reactivity and can negatively affect the system’s performance in its final application.
Various methods were employed to investigate the storage stability of effervescent tablets or powder mixtures in an open storage test under harsh conditions (30°C, 60% relative humidity).
The powder mixtures and tablets were visually assessed. Tablets containing regular citric acid showed surface roughening and foaming (carbon dioxide formation), whereas the surface of CITROCOAT® EP tablets did not exhibit any visible changes (Figure 2).
Figure 2: Visual assessment of effervescent tablets after being stored open for 88 days under harsh conditions (30°C, 60% relative humidity), with foaming and caking visible for regular citric acid tablets and no change visible for tablets with CITROCOAT® EP.
The change in mass resulting from the formation of CO2 during the storage period was also measured. CITROCOAT® EP tablets showed the smallest mass changes, followed by CITROCOAT® N, indicating improved storage stability of effervescent systems.
Reactivity of effervescent powders after storage The reduction in CO2 formation due to premature reaction during open storage was also evaluated. The volume of CO2 produced by the mixture of regular citric acid and sodium bicarbonate fell by almost half (47%) after 88 days of open storage under harsh conditions (30°C, 60% relative humidity). In contrast, the functional acids exhibited greater stability in the effervescent mixture – Citric Acid DC produced 28% less CO2, CITROCOAT® N produced 22% less CO2 and CITROCOAT® EP produced only 9% less CO2 after storage.
Application example
Ensuring good storage stability and rapid dissolution with effervescent formulations is challenging, especially if hygroscopic components are included. Beverage tablets containing natural extracts and fruit powders are particularly sensitive to moisture and tend to clump together. This can impact tablet hardness, dissolution time, and storage stability.
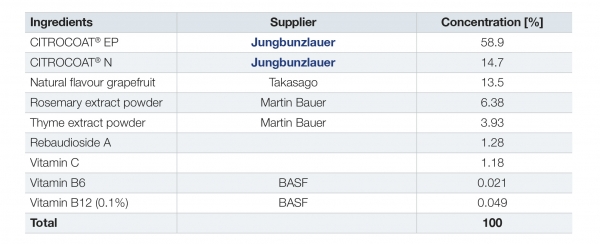
A sample beverage tablet formulation (Table 1) was developed and compared to a formulation using regular citric acid and sodium bicarbonate. The recipe includes CITROCOAT® EP as a ready-to-use effervescent compound and CITROCOAT® N for pH control.
Table 1: Recipe for effervescent beverage tablets, with ingredients and their suppliers.
Tablets containing CITROCOAT® EP required 23% less force to achieve the same hardness as tablets with regular citric acid. Furthermore, when dissolved in water at room temperature without stirring, tablets with CITROCOAT® EP dissolved 24% faster than those using regular citric acid.
The stability of tablets during storage is an important consideration for consumers, as it impacts packaging requirements. To assess this, accelerated stability tests were conducted by exposing tablets to open storage in a climate chamber at 30°C and 60% relative humidity. The Jungbunzlauer tablets were compared to a benchmark product in this test.
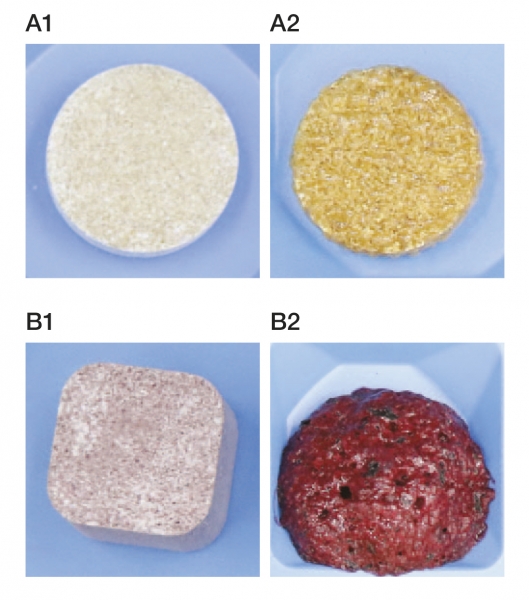
After just one day of storage, noticeable changes were observed in the market samples (Figure 3, image B). The tablets had almost completely dissolved and had lost their structure, with visible bubbles indicating the release of carbon dioxide and moisture absorption. On the other hand, the tablets with CITROCOAT® EP had slightly darkened surfaces over the same period (Figure 3, image A), suggesting some moisture absorption. However, these tablets maintained their shape, and the absence of bubbles indicated that no effervescent reaction had taken place.
Figure 3: Beverage tablets (A: recipe with CITROCOAT® EP; B: market sample) before (1) and after (2) being stored at 30°C and 60% relative humidity for one day.
As a result, beverage tablets made with CITROCOAT® EP can be stored with less packaging or more sustainable packaging. This is because they exhibit greater stability under harsh environmental conditions and have a longer shelf life compared to tablets using regular citric acid.
References
1. Awad, A.; Trenfield, S.; Basit, A.; (2020). Solid oral dosage forms. Remington: The Science and Practice of Pharmacy, 23rd ed.; 333-358.
2. Testbook Edu Solutions. What is Brisk Effervescence? – Check Definition, Chemical Reaction & Important Questions 2023.
3. Wells, M. L.; Wood, D. L.; Sanftleben, R.; et al. (1997). Potassium carbonate as a desiccant in effervescent tablets. Int. J. Pharm. 152(2):227–235.